Properties of Water Summary Note
View
5 Unique Properties of Water
Due to polarity water exhibits:
- Cohesion (sticks to self)
- Adhesion (sticks to other things)
- High specific heat capacity / high vaporization
- Universal solvent
- Less dense as a solid
Unique Properties of Water & How Polarity is Involved
- Cohesion --> H-bonds
- Adhesion --> H-bonds / dipole attractions
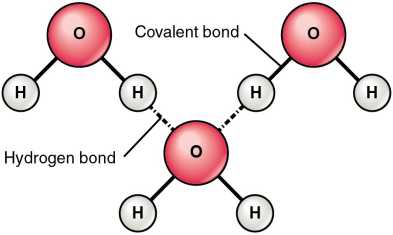
- High specific heat capacity / high vaporization --> H-bonds
- Universal solvent --> Polarity
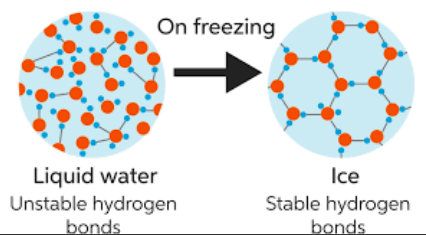
- Less dense as a solid --> H-bonds & bent shape
Which property?
- Water comes off of the tap in a stream --> Cohesion
- Humans sweat to cool their core body temperature --> High heat capacity
- Water boils at 100℃ & methane (CH4) at -161℃ --> High heat vaporization
- Water "climbs" up pant legs on a rainy day --> Adhesion
- Lakes freeze from the top to the bottom --> Less dense as solid
- Sugar is a solute in Coke & Pepsi --> Universal solvent
Video: Properties of Water Lab
Last modified: Monday, 17 February 2025, 3:59 PM