Lesson 5.9 - Introduction to Nuclear Physics
VIDEO LESSON Part 2 (19:53 min)
Video is not accessible in some countries. If you cannot view it, you might need VPN to do so.
A VERY QUICK INTRODUCTION TO NUCLEAR PHYSICS
We will discuss:
- Nuclear Decay
- Radioactive Dating
- Nuclear Fission and Fusion
This is by no means an exhaustive look at nuclear physics, just a very quick overview.
So far, our discussion on subatomic structure has been limited to electrons and their quantum mechanical behaviour. To descend further into the atom, things become even stranger. Scientist still don't yet have a complete understanding of the full laws governing this tny yet dense region: the nucleus.
Consider this, if an atom is the size of a classroom with the electrons smeared out in circular and 'dumbell' shaped clouds, the nucleus would be approximately the size of a grain of sand in the centre of the room. BUT it would contain ALL of the weight of the entire room in that small volume.
The nucleus is what is responsible for element being that type of element. We change the structure of the nucleus, we have elements changing from one to another. The ancient alchemist dreamed of "transmuting" elements, turning lead into gold. In nucleus physics it actually happens.
NUCLEAR DECAY
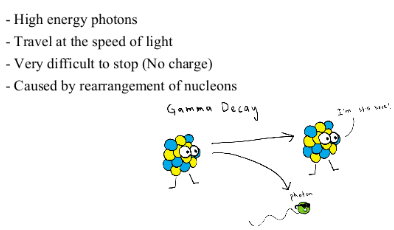
It was discovered in the beginning of the 20th century that some materials emit radiation when they decompose into different elements. We call this radioactivity.Three types of radioactive emissions

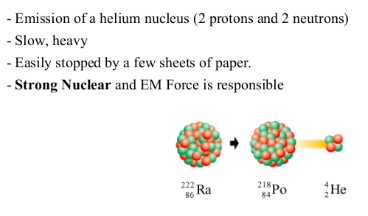
ALPHA DECAY
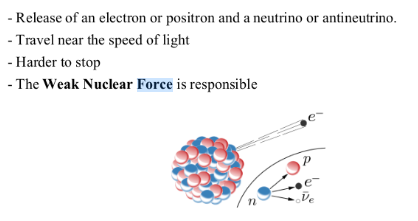
BETA DECAY
GAMMA DECAY
RADIOACTIVE DATING
A radioactive material breaks down at a rate proportional to the amount left. This means that the amount left in a substance decreases exponentially.Example: Carbon-14
Nitrogen in the atmosphere is converted into Carbon-14 when struck by a cosmic ray. Living things breathe it in and it becomes bound up in their bodies. When the animal dies no more C-14 can be absorbed. It decays so that after every 5739 years half of the original is left.
The amount of C-14 left in a fossil or other dead organism can be modelled by:
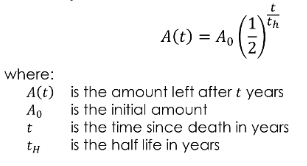
EXAMPLE PROBLEM #1
If a material is found to have 37% of the C-14 it is supposed to have in it, how long has it been since it died?SOLUTION:
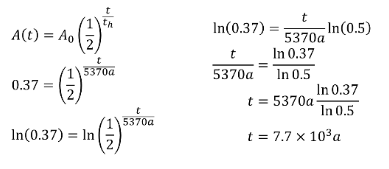
ENERGY AND NUCLEAR DECAY
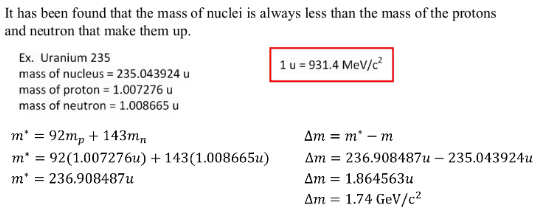
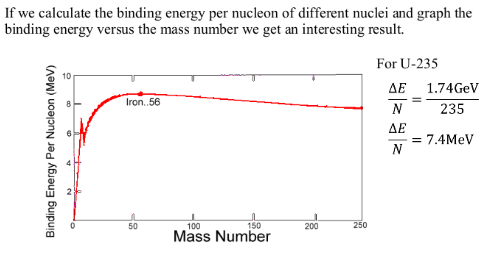
Large nuclei (mass greater than 56) tend to break apart, undego fission, to increase the binding energy. The higher the binding energy the more stable the nuclei.
Small nuclei (mass less thatn 56) tend to combine with other nuclei, undergo fusion, to increase the binding energy. The higher the binding energy the more stable the nuclei.
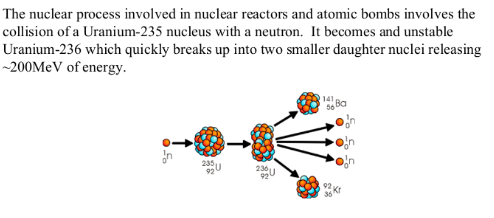
AN EXAMPLE OF NUCLEAR FISSION

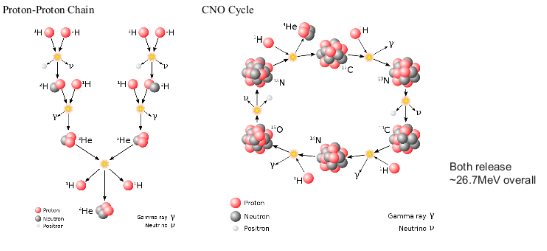
NUCLEAR FUSION


SOLAR FUSION
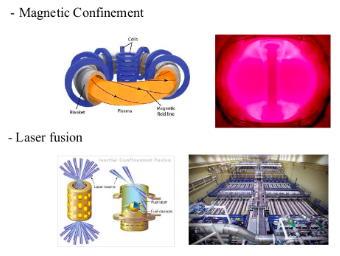
EARTHBOUND FUSION