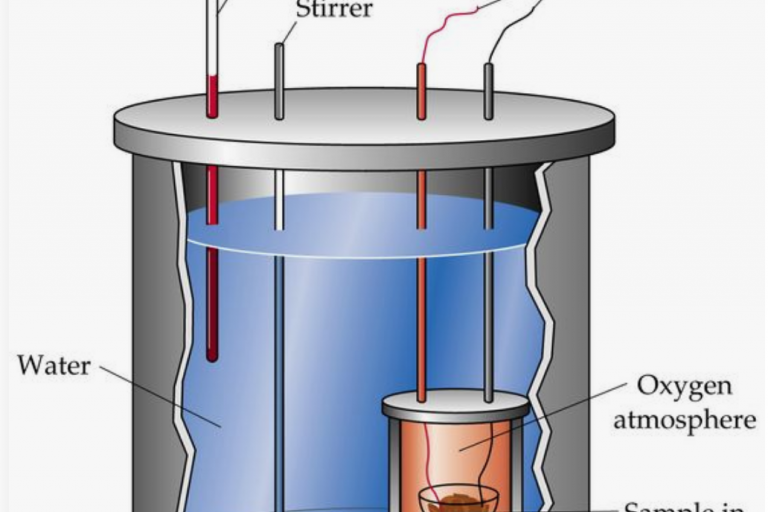
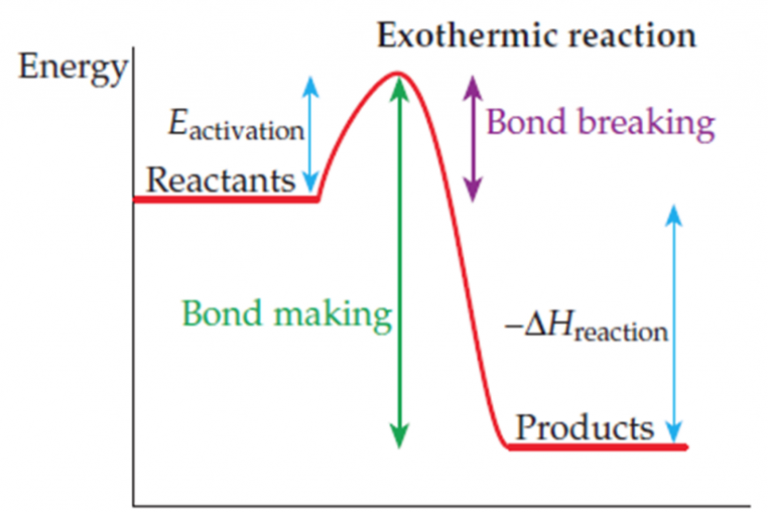
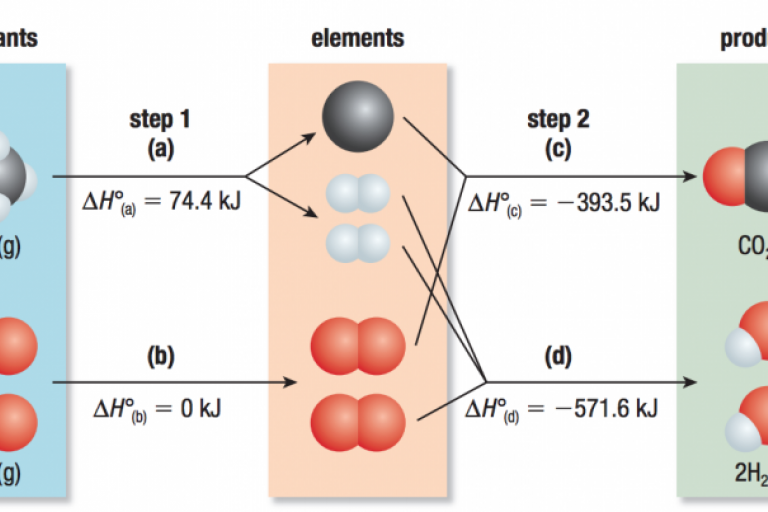
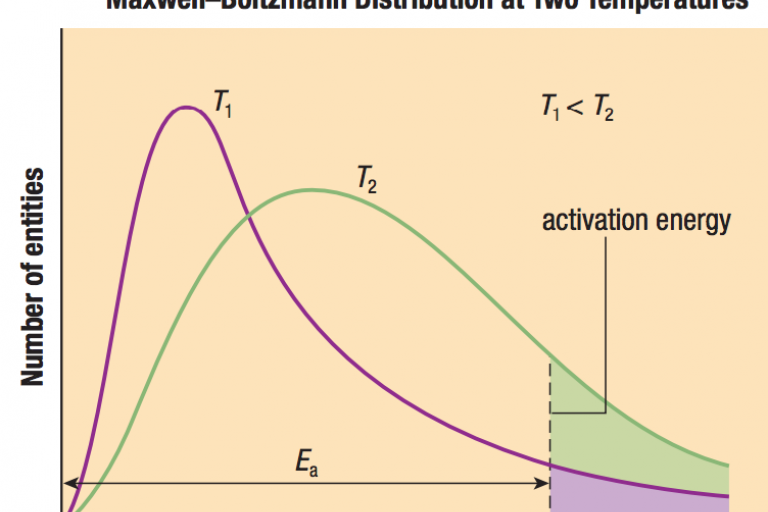
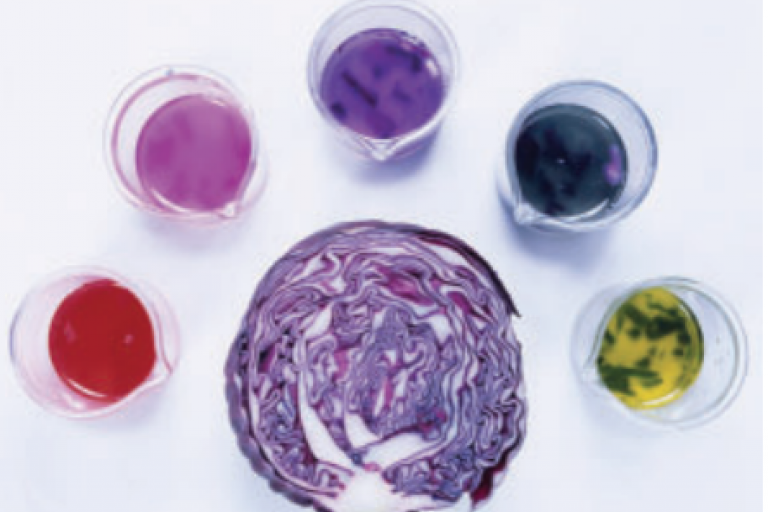
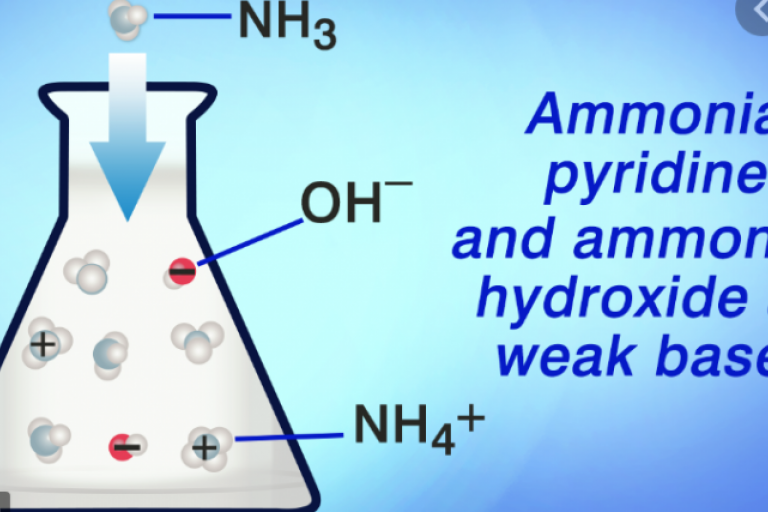
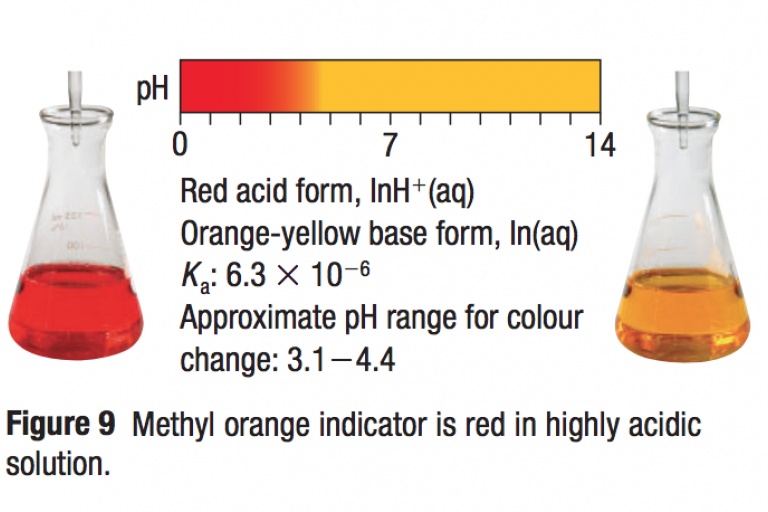
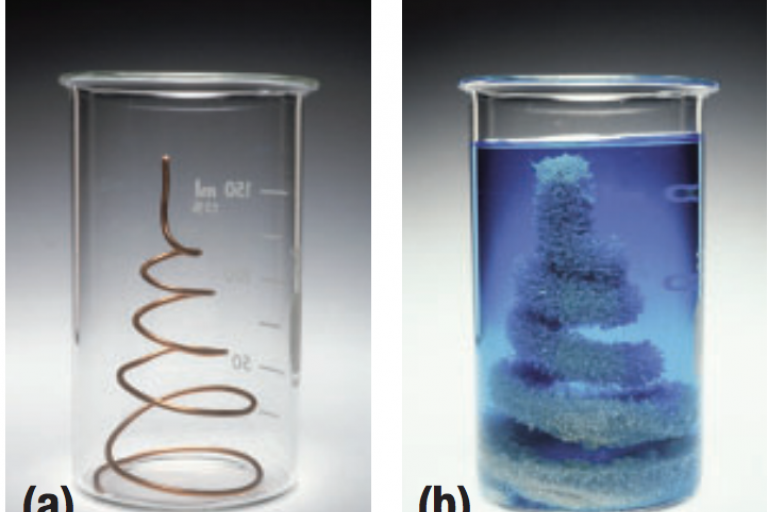
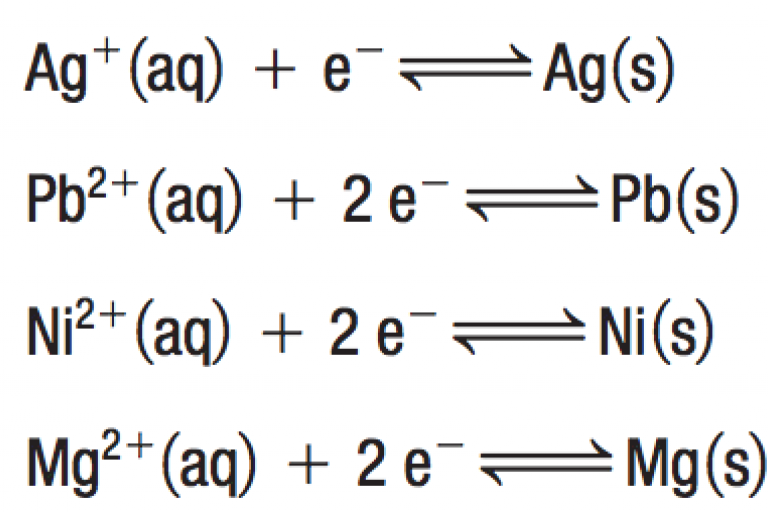Welcome to Grade 12 Chemistry SCH4U!


Class Schedule: Monday to Friday 8:45am - 12pm EST.
- Office hour: Every Friday 8 - 9 am EST.
- Teacher's email:
- For Tech support email Fan Wu: fanw@edu4u.ca
- Moodle Maintenance: A reminder that every other Friday 5 pm - 11:59 pm (EST), Moodle is unavailable as it is undergoing maintenance. Ensure you have all the work you need downloaded or that you complete your tasks outside of this time
Grade 11 Textbook: https://www.creativebookpublishing.ca/books/McGraw-Hill-Ryerson-Chemistry-11.pdf
Grade 12 Textbook: https://www.creativebookpublishing.ca/books/Nelson-Chemistry-12.pdf